A Barrie businessman set to distribute a rapid COVID-19 serology test across Canada has been frustrated by efforts to have it approved by Health Canada.
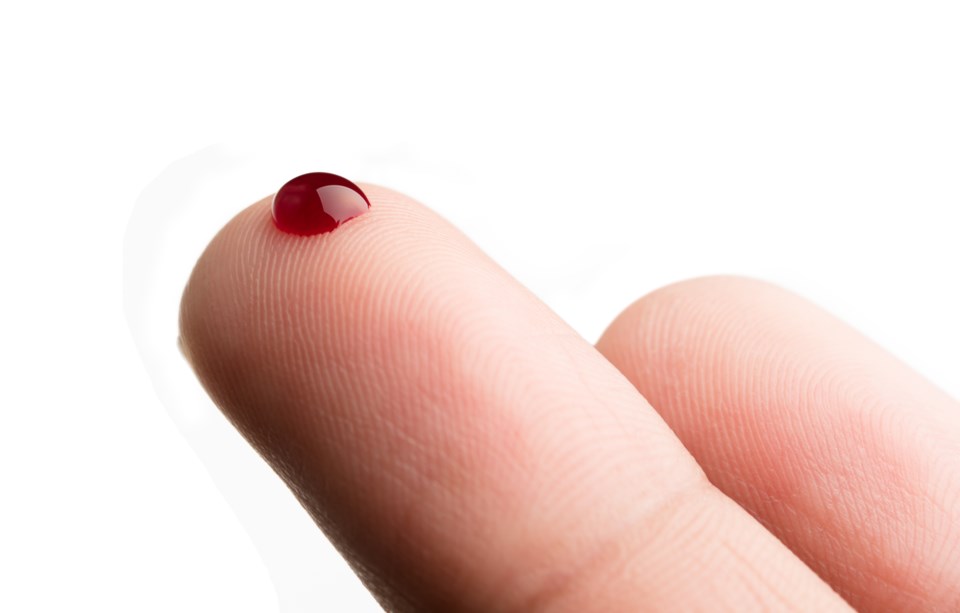
Helix 19 is a serology, or finger-prick blood test, that provides results within three minutes and Peter Lorimer, of Loraday Environmental Products Ltd. in Barrie, is set to distribute it throughout Canada.
The test shows if the individual has the virus, has had the virus or is negative.
But he says two applications through the Health Canada portal hasn’t got the product any closer to approval.
“They have yet to be looked at or put on the list” of those to be looked at, said Lorimer. “All of the clinical data meets what they’re looking for.”
The test has already made it through the regulatory process in the United States, where Lorimer said they’ve received their U.S. Food and Drug Administration (FDA) emergency use authorization number and are ready to sell to medical services and professionals there.
He said it is also approved for usage in the European Union and has been widely used in China where it was produced.
Because it is a quick test specific to COVID-19, there is potential to get ahead of the virus in Canada, he said.
Results from the more common throat swab tests currently being conducted takes several days.
“If you can do a massive testing and you can find out where your hotspots are across Canada, you can isolate the hotspots and then let the rest of the economy get back to work,” he said.
Barrie-Innisfil MP John Brassard has raised the issue of the approval process related to serology testing in the House of Commons and sees it key to controlling the situation.
“Testing, tracing and isolation are going to be critical to widely opening up of the economy and to prevent the spread of this,” said Brassard. “I had the test done and it came back negative. It takes literally three minutes.”
That the test has been accepted by other jurisdictions is some indication that perhaps the Canadian government should be paying attention to it as well, he said.
“It’s reasonable to me that this type of technology… could really assist,” he said. “The best way is through serological testing because we’ve seen even through the mucus testing a lot of false positives…. Blood through the serological testing seems to be the way to go.”
Health Canada, which regulates the sale and import of commercial testing devices, said most applications for COVID-19 tests are either nucleic acid-based or serology-based. It publishes a list of applications for testing devices that are currently under evaluation and Helix 19 does not appear on that list.
Loraday Environmental Products started 26 years ago with an environmental absorbent and expanded to distribute other related products across the country. That included personal protection equipment N-95 masks and when SARS arrived in Ontario.